Download search results¶
Select columns to include and download a CSV file with the search results.
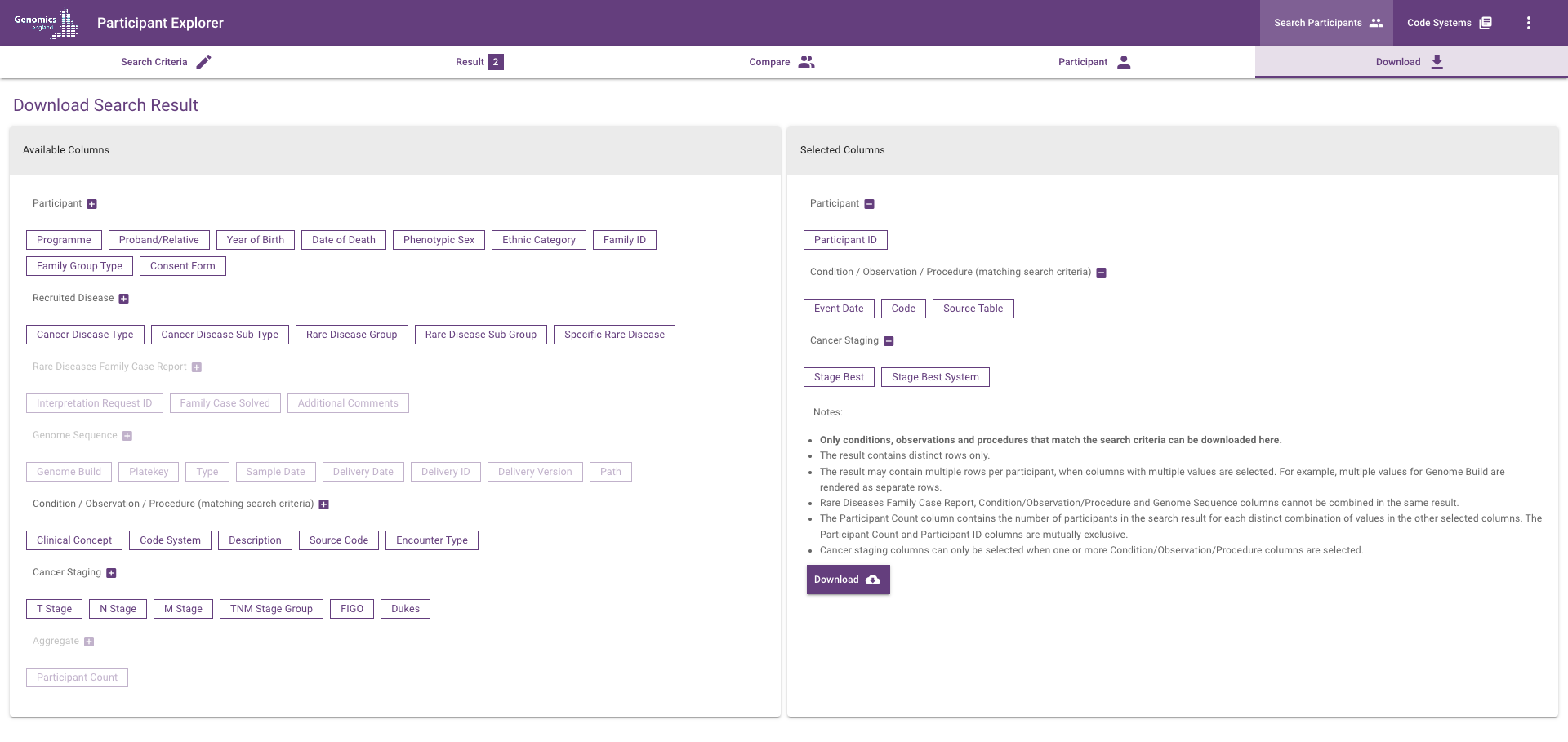
Participant columns¶
Please refer to the Participant view page for details.
Recruited disease columns¶
Typically a participant is recruited for a single disease, but multiple recruited diseases are possible (resulting in multiple rows per participant).
| Column | Notes |
|---|---|
| Rare Disease Group | The recruited rare disease group |
| Rare Disease Sub Group | The recruited rare disease sub group |
| Specific Rare Disease | The specific recruited disease (same as displayed in the search results) |
| Cancer Type | The recruited cancer type |
| Cancer Sub Type | The recruited cancer sub type |
Rare diseases family case report columns¶
Details of the clinical report delivered for a rare diseases case from the GMC exit questionnaire table in LabKey.
In most cases there is only a single report, but multiple reports are possible (resulting in multiple rows per participant).
| Column | Notes |
|---|---|
| Interpretation Request ID | The Interpretation Request contains all of the information needed to display and clinically annotate a case. If new data are added (such as an additional family member or gene panel), the case will be re-interpreted and the interpretation request id will be updated. |
| Family Case Solved | Have the results reported explained the genetic basis of the family’s presenting phenotype(s)? (Yes / No / Partially / Unknown) |
| Additional Comments | Comments regarding the report |
Genome sequence columns¶
Please refer to the Participant View page for column details.
Genome sequence columns will generate multiple rows for participants with multiple genome sequence deliveries.
Condition/observation/procedure/drug columns¶
Separate rows will be generated for conditions, observations, procedures and drugs that matched the search criteria. This corresponds to the highlighted rows in the timeline, and includes matches based on subsumption (when descendant codes are implicitly included in the search).
These columns are only available when using the search-by-clinical-concept option.
Multiple rows will be generated for participants with multiple (different) codes that matched the search criteria.
| Column | Notes |
|---|---|
| Event date | The date of the condition, observation, procedure or drug. |
| Clinical Concept | Whether the code represents a condition, observation, procedure or drug. |
| Code System | The name of the code system the code belongs to, e.g. "ICD-10". |
| Code | The code as displayed in the Participant Explorer. This is a normalised version of the source code. E.g. ICD10 code "E149-" is normalised to "E14.9". See Data in the Participant Explorer for more details. |
| Description | The description / display value for the code. |
| Source Code | The code as it appears in the source data. |
| Source Table | The name of the table (in the research data release) from which the condition, observation, procedure or drug information was obtained. |
| Encounter Type | The type of encounter during which the condition, observation, procedure or drug occurred. |
Cancer Staging Columns¶
Additional columns for staging data associated with cancer diagnoses.
These columns are available when using the search-by-clinical-concept option, and can only be selected after selecting at least one column from the "Condition/observation/procedure/drug" category.
| Column | Notes |
|---|---|
| Stage Best | Stage (Best) (NCRAS) |
| Stage Best System | The staging system used for Stage Best (NCRAS) |
| T Stage | The T stage category |
| N Stage | The N stage category |
| M Stage | The M stage category |
| TNM Stage Group | The TNM stage group |
| FIGO | The FIGO stage |
| Dukes | The Dukes stage |
The availability of stage values depend on the source table of the diagnosis data point. See Data in the Participant Explorer for more details.
Aggregate columns¶
| Column | Notes |
|---|---|
| Participant Count | The number of distinct participants in the search result with the combination of values in the other selected columns. This column will be disabled (greyed out) if the Participant ID column is selected, and vice versa. |
Notes
- Only conditions, observations, procedures and drugs that match the search criteria can be downloaded here.
- The result contains distinct rows only.
- The result may contain multiple rows per participant, when columns with multiple values are selected. For example, multiple values for Genome Build are rendered as separate rows.
- Rare Diseases Family Case Report, Condition/Observation/Procedure/Drug and Genome Sequence columns cannot be combined in the same result.
- The Participant Count column contains the number of participants in the search result for each distinct combination of values in the other selected columns. The Participant Count and Participant ID columns are mutually exclusive.
Export from TRE¶
Tables exported from Participant Explorer which include participant identifiers count as identifiable data and cannot be exported from the RE. Tables which include only counts may be able to be exported if all counts are greater than five. You should use Airlock for any exports and not copy these by hand.